Answer:
18.42 years.
Explanation:
We have been that the half-life of a certain substance is 25 years. We are asked to find time taken by substance to decay to 60% of its original amount.
We will use formula
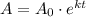 to solve our problem.
to solve our problem.
First of all, we will find value of k using
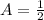 ,
,
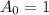 and
and
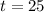 .
.
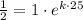
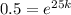
Now we will take natural log of both sides of equation.
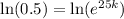
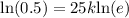
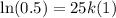
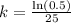
Our function would be
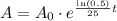 .
.
Now we have
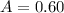 and
and
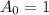 .
.
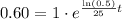
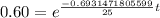
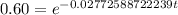
Now we will take natural log on both sides.
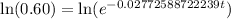
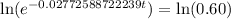
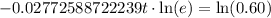
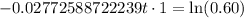
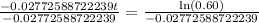
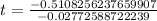
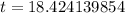
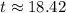
Therefore, it will take approximately 18.42 years for a sample of this substance to decay to 60% of its original amount