Answer:
The molarity of the CoBr2•4H2O solution is 7.64 × 10-2 M
Step-by-step explanation:
Cobalt (II) bromide tetrahydrate
• Cobalt - A transition metal with Roman numeral (II) → charge: +2 → Co2+
• Bromide - anion from group 7A → -1 charge → symbol: Br-
• Tetrahydrate- tetra- means 4 and hydrate is H2O
The chemical formula of the compound is: CoBr2•4H2O
We then need to determine the number of moles of CoBr2•4H2O since this is the only information missing for us to find molarity. Notice that the volume of the solution is already given.
We’re given the mass of CoBr2•4H2O. We can use the molar mass of CoBr2•4H2O4 to find the moles.
•The molar mass of CoBr2•4H2O is:
CoBr2•4H2O
1 Co x 58.93 g/mol Co = 58.93 g/mol
2 Br x 79.90 g/mol Br = 159.80 g/mol
8 H x 1.008 g/mol H = 8.064 g/mol
4 O x 16.00 g/mol O = 64.00 g/mol
________________________________________
Sum = 290.79 g/ mo
The moles of CoBr2•4H2O is:
= 10.0 g CoBr2•4H2O x
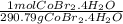
= 0.0344 mol CoBr2•4H
We know that the volume of the solution is 450 mL.
We can now calculate for molarity:
Convert mL to L → 1 mL = 10-3 L
Formula:
Molarity (M)= Mole of solute / Liters of solution
= 0.0344 mol CoBr2•4H / 450 mL x 1 ml / 10^ -3 L
= 0.0764
= 7.64 × 10-2 mol/L