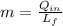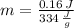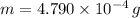Answer:
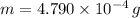
Step-by-step explanation:
By means of the First Law of Thermodynamics, which is a generalization of the Principle of Energy Conservation, the kinetic energy of the hammer is dissipated by the hammer in the form of heat, which melts the ice. That is to say:
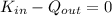
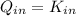
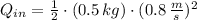
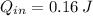
Let consider that ice melts a 0 °C and 1 atm, so that heat is entirely latent. Hence:
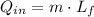
The melt mass of ice by a single blow is: