Answer:
Part A:
The proton has a smaller wavelength than the electron.
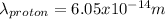 <
<
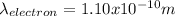
Part B:
The proton has a smaller wavelength than the electron.
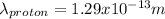 <
<
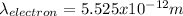
Step-by-step explanation:
The wavelength of each particle can be determined by means of the De Broglie equation.
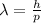 (1)
(1)
Where h is the Planck's constant and p is the momentum.
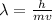 (2)
(2)
Part A
Case for the electron:
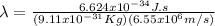
But
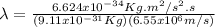
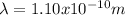
Case for the proton:
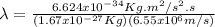
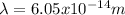
Hence, the proton has a smaller wavelength than the electron.
Part B
For part b, the wavelength of the electron and proton for that energy will be determined.
First, it is necessary to find the velocity associated to that kinetic energy:
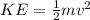
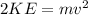
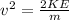
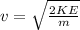 (3)
(3)
Case for the electron:
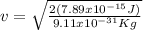
but
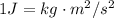
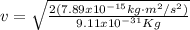
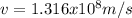
Then, equation 2 can be used:
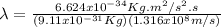
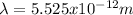
Case for the proton :
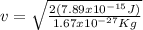
But
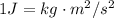
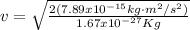
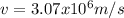
Then, equation 2 can be used:
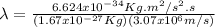
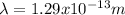
Hence, the proton has a smaller wavelength than the electron.