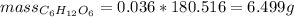Answer:
The mass of sugar glucose is 6.499 g
Step-by-step explanation:
Data given:
mass of CO₂ = 9.51 g
molar weight of CO₂ = 44.01 g/mol
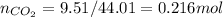
The reaction is:
C₆H₁₂O₆ + 9O₂ = 6CO₂ + 6H₂O
According the reaction:
1 mol of C₆H₁₂O₆ ------------- 6 moles of CO₂
X mol of C₆H₁₂O₆ ------------- 0.216 moles of CO₂
Clearing X:
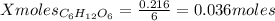
molar weight of C₆H₁₂O₆ = 180.516 g/mol
The mass of glucose is: