Answer:
pH = 5.58
Step-by-step explanation:
Given that :
Citric acid is a triprotic acid with the formula
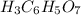 . Tripotic acids are acids that have the tendency to yield three protons per molecule during the phases of dissociation, therefore they have three pka.
. Tripotic acids are acids that have the tendency to yield three protons per molecule during the phases of dissociation, therefore they have three pka.
For Citric acid; the pka values are:
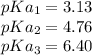
So, the pH of sodiummonohydrogencitrate with the chemical formula
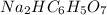 which can act as an acid and a base (i.e it is said to be amphoteric) can be calculated as :
which can act as an acid and a base (i.e it is said to be amphoteric) can be calculated as :
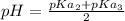
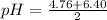
pH = 5.58
Therefore, the approximate pH of a solution of sodium monohydrogencitrate = 5.58