Answer:
50%
Step-by-step explanation:
Formula of this hydrate is given as:
Sodium bicarbonate octahydrate = NaHCO₃ . 8H₂O;
To find the percent by mass of water;
- Sometimes it is good to know the percent by mass of elements or groups present in a compound.
- Find the formula mass by summing the atomic masses.
- Determine the percentage composition of an atom or group by expressing as a percentage of the formula mass.
Formula mass of NaHCO₃ . 8H₂O;
= 23 + 1 + 12 + 3(16 ) + 8[2(1) + 16]
= 228g/mol
for the 8H₂0 = 8(18) = 144
%H₂O =
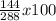 = 50%
= 50%