Answer: 249 grams
Step-by-step explanation:
The balanced chemical equation is :
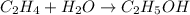
As water is present in excess , it is called as excess reagent and ethylene is the limiting reagent.
According to stoichiometry:
1 mole of ethylene produces = 1 mole of ethanol
Thus 5.40 mole of ethylene produces =
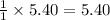 mole of ethanol
mole of ethanol
Mass of ethanol =
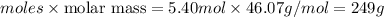
Thus 249 g of ethanol are produced from 5.40 mol of ethylene