Answer:
number of moles of
 =4.16mol
=4.16mol
Step-by-step explanation:
experiment data:
temperature=
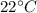 =273+22 k=295k
=273+22 k=295k
pressure=1.007 atm
volume is missing so assuming volume-=100L
using ideal law relationship
ideal gas means gas which occupies negligible space and there is no interaction between the molecules of gases.
PV=nRT
put all the experimental data, we get
n=4.15 mol
number of moles of
 =4.16mol
=4.16mol