Answer: pH of the given solution is 8.7.
Step-by-step explanation:
For the buffer mixture, initial pOH will be given as follows.
pOH =
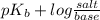
=
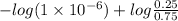
=
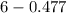
= 5.523
When 0.05 mol NaOH is added to this buffer solution then concentration of species present will be as follows.
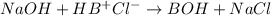
0.05 mol 0.25 0.75 0
0 0.20 (0.75 + 0.25) 0.05
Hence, the volume of solution will be 1 liter.
[BOH] =
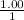 , [Salt] =
, [Salt] =
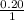
So, pOH =
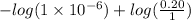 [/tex]
[/tex]
= 6 - 0.69
= 5.30
Now, we will calculate the pH as follows.
pH = 14 - 5.30
= 8.7
Thus, we can conclude that pH of the given solution is 8.7.