Answer:
0.0289 x 10⁻¹⁹kg
Step-by-step explanation:
The de Broglie wavelength, λ, of a particle varies inversely with the momentum (the product of the mass, m, and velocity, v) of the particle. i.e
λ ∝
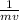
λ =
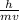 ------------------------(i)
------------------------(i)
Where;
h = constant of proportionality called Planck's constant = 6.626 x 10⁻³⁴Js
From the question;
λ = 0.950fm = 0.950 x 10⁻¹⁵m
v = 241ms⁻¹
Substitute these values into equation (i) as follows;
0.950 x 10⁻¹⁵ =
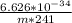
Solve for m;
m =
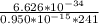
m = 0.0289 x 10⁻¹⁹kg
Therefore, the maximum mass of the object is 0.0289 x 10⁻¹⁹kg