Answer:
123 is the equilibrium constant for the reaction.
Step-by-step explanation:
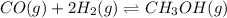
Equilibrium concentration of reactants :
![[CO]=0.115 M,[H_2]=0.116 M](https://img.qammunity.org/2021/formulas/chemistry/college/ighhe2dpc2m9c3k5cvfcx6epw618v701ae.png)
Equilibrium concentration of products:
![[CH_3OH]=0.190 M](https://img.qammunity.org/2021/formulas/chemistry/college/hjt75tegacvkm2t70325zpjc29rr6odyi9.png)
The expression of an equilibrium constant is given by :
![K_c=([CH_3OH])/([CO][H_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/221676sk33k3xtvein3nbay5z2mn8nt4yi.png)
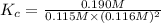
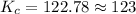
123 is the equilibrium constant for the reaction.