Answer:
1.645 moles of excess reactant that is of magnesium metal are left over.
Step-by-step explanation:
Moles of magnesium metal = 3.29 mol
Moles of HCl = 3.29 mol
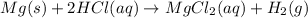
According to recation, 2 moles of HCl reacts with 1 mol of magnesium metal, then 3.29 moles of HCl will react with :
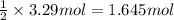 of magnesium metal
of magnesium metal
Moles of HCl left = 3.29mol - 3.29 mol = 0
Moles of magnesium metal left = 3.29 mol - 1.645 mol = 1.645 mol
1.645 moles of excess reactant that is of magnesium metal are left over.