(b) 12.7 L
Step-by-step explanation:
Given:
Volume, V₁ = 12 L
Pressure, P₁ = 760 mm Hg (at STP)
Temperature, T₁ = 273 K (at STP)
Pressure, P₂ = 720 mm Hg
Temperature, T₂ = 0°C = 273 K
Volume, V₂ = ?
We know:
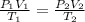
Substituting the value we get:
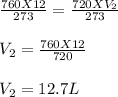
Therefore, V₂ is 12.7 L