The new volume is 15.59L
Step-by-step explanation:
Given:
Initial volume, V₁ = 4L
Initial number of moles, n₁ = 0.975 mol
Final number of moles, n₂ = 3.8 mol
New volume, V₂ = ?
According to Avogadro's law:
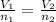
Substituting the values we get:
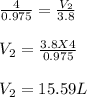
Therefore, the new volume is 15.59L