Answer:
4.48 grams is the mass of potassium hydroxide that the chemist must weigh out in the second step.
Step-by-step explanation:
The pH of the solution = 13.00
pH + pOH = 14
pOH = 14 - pH = 14 - 13.00 = 1.00
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
![1.00=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/rifyfzv54w53sfb0ts6kio77c7fjblqc9t.png)
![[OH^-]=10^(-1.00) M=0.100 M](https://img.qammunity.org/2021/formulas/chemistry/college/60ce1zq6asgzz9pe2lfx3687itdebhj1fn.png)
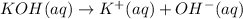
![[KOH]=[OH^-]=[K^+]=0.100 M](https://img.qammunity.org/2021/formulas/chemistry/college/1rw7iux2yzr9w9okpqmu0cwxhge38k50jk.png)
Molariy of the KOH = 0.100 M
Volume of the KOH solution = 800 mL= 0.800 L
1 mL = 0.001 L
Moles of KOH = n
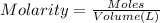
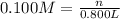
n = 0.0800 mol
Mass of 0.0800 moles of KOH :
0.0800 mol × 56 g/mol = 4.48 g
4.48 grams is the mass of potassium hydroxide that the chemist must weigh out in the second step.