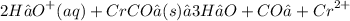Step 1: Write the balanced "molecular equation:
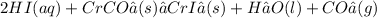
Step 2: Carbon dioxide and water are written in molecular form. Consult the solubility and net ionic equation rules on the information page to determine which of the other substances will dissociate:
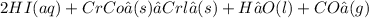
yes no yes
Step 3: Dissociate all soluble salts, strong acids, and strong bases (except calcium hydroxide), Leave together all "not soluble" salts and weak acids or bases:
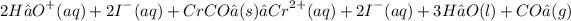
Step 4: Cross out "spectator lons that appear on both sides of the reaction (these lons do not participate in the chemistry) and rewrite the "net" reaction using the smallest possible coefficients: