Answer:
8.60 *
 atoms N2
atoms N2
Step-by-step explanation:
We want to convert grams to moles and then moles to atoms.
First, we convert grams of nitrogen gas (which is N2) to moles. To do so, we need the molar mass of N2, which is just 14.01 * 2 = 28.02 g.
40 g N2 *
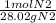 = 1.43 mol N2
= 1.43 mol N2
Now, we need to convert moles to atoms by using Avogadro's number, which is
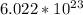 :
:
1.43 mol N2 *
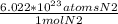 = 8.60 *
= 8.60 *
 atoms N2
atoms N2
Thus, the answer is 8.60 *
 atoms N2.
atoms N2.