The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen.
Suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924 liters per second of methane are consumed when the reaction is run at 261°C and 0.96atm. Calculate the rate at which dihydrogen is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits.
Answer: The rate at which dihydrogen is being produced is 0.12 kg/sec
Step-by-step explanation:
The balanced chemical equation is ;
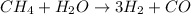
According to ideal gas equation:

P = pressure of gas = 0.96 atm
V = Volume of gas = 924 L
n = number of moles
R = gas constant =
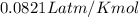
T =temperature =
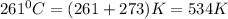
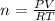
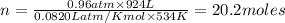
According to stoichiometry:
1 mole of methane produces = 3 moles of hydrogen
Thus 20.2 moles of methane produces =
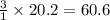 moles of hydrogen
moles of hydrogen
Mass of hydrogen =
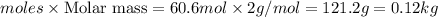
Thus the rate at which dihydrogen is being produced is 0.12 kg/sec