Answer:
The amount that oxidized to NiO(OH) is = 1.46 gm
Step-by-step explanation:
Given data
Current I = 0.35 A
Time taken = 145 min
We know that charge
Q = I t
Q = 0.35 × 145 × 60
Q = 3045 C
Faraday's constant = 96500 C
No. of moles of electron
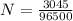
N = 0.03155
1 mol of
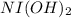 is oxidized by 2 moles of electrons, so no. of moles can be oxidized is
is oxidized by 2 moles of electrons, so no. of moles can be oxidized is
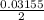 = 0.015775 moles
= 0.015775 moles
Now convert this moles into gm by multiplying 92.708
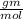
0.015775 × 92.708 = 1.46 gm
Therefore the amount that oxidized to NiO(OH) is = 1.46 gm