Answer: The mass of AgI in the precipitate is 9.55 g.
Step-by-step explanation:
The chemical equation for this reaction is as follows.
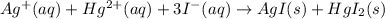
So, we will calculate the moles of added as follows.
Moles = Molarity × Volume
= 1.71 \times 0.100
= 0.171 moles added
Let us assume that consumes x moles of and consumes 2x moles of
3x = 0.122 mol
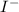
or, x =
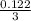
= 0.0407 mol
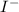
So,
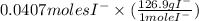
= 5.16 g
Hence, the formula AgI the mole ratio of to is 1:1.
0.0407 moles
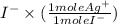
= 0.0407 moles
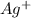
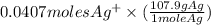
= 4.39 g
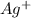
Therefore, we will calculate the mass of AgI as follows.
Mass of AgI = mass of
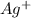 + mass of
+ mass of
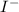
= 5.16 + 4.39
= 9.55 g AgI
Therefore, we can conclude that the mass of AgI in the precipitate is 9.55 g.