Answer : The half-life of the reaction in seconds is, 244
Explanation :
The expression used for zero order reaction is:
![t_(1/2)=([A_o])/(2k)](https://img.qammunity.org/2021/formulas/chemistry/college/ltf2hgs1nz11xu3q7ywtfzptaays9qqdyb.png)
where,
 = half-life of the reaction = ?
= half-life of the reaction = ?
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = initial concentration = 5.90 M
= initial concentration = 5.90 M
k = rate constant = 0.725 M/min
Now put all the given values in the above formula, we get:
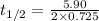
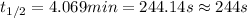
conversion used : (1 min = 60 s)
Thus, the half-life of the reaction in seconds is, 244