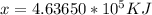Complete Question
To make use of an ionic hydrate for storing solar energy, you place 422.0 kg of sodium sulfate decahydrate on your house roof. Assuming complete reaction and 100% efficiency of heat transfer, how much heat (in kJ) is released to your house at night? Note that sodium sulfate decahydrate will transfer 354 kJ/mol.
Answer:
The amount of energy released is
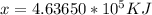
Step-by-step explanation:
Number of moles is mathematically represented as
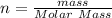
substituting
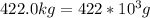 for mass of sodium sulfate decahydrate(
for mass of sodium sulfate decahydrate(
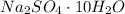 ),
),
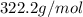 (This value is a constant )for the molar mass of sodium sulfate decahydrate
(This value is a constant )for the molar mass of sodium sulfate decahydrate
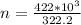
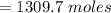
From the question we are told that
1 mole of sodium sulfate decahydrate generates
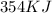 of energy
of energy
So 1309.7 mole would generate x
Now stating the relation mathematically
1 mol → 354KJ
1309.7 mol → x
=>