Answer:
![[SO_2Cl_2] = 0.09983 M](https://img.qammunity.org/2021/formulas/chemistry/college/xlc8magz5pogpndhuf9ldx1y6e8saprxds.png)
Step-by-step explanation:
Write the balance chemical equation ,
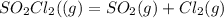
initial concenration of
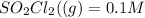
lets assume that degree of dissociation=
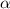
concenration of each component at equilibrium:
![[SO_2Cl_2] = 0.1-0.1\alpha](https://img.qammunity.org/2021/formulas/chemistry/college/day6s5aq3tpyvqwjgz9krtjwawukymtaft.png)
![[SO_2] = 0.1\alpha](https://img.qammunity.org/2021/formulas/chemistry/college/5fuqtkgzlkynq9qavnj1m06wecqv93gdz7.png)
![[Cl_2] = 0.1\alpha](https://img.qammunity.org/2021/formulas/chemistry/college/hlwzw1htwqevvofo2k25kjyvihpwspg0ha.png)
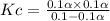
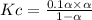
as
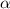 is very small then we can neglect
is very small then we can neglect

therefore ,
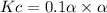
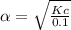
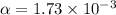
Eqilibrium concenration of
![[SO_2Cl_2] = 0.1-0.1\alpha = 0.1-0.1* 0.00173](https://img.qammunity.org/2021/formulas/chemistry/college/def8iu9iowzfssjy5wbtfi2c8ac0xeg48f.png)
![[SO_2Cl_2] = 0.09983 M](https://img.qammunity.org/2021/formulas/chemistry/college/xlc8magz5pogpndhuf9ldx1y6e8saprxds.png)