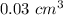For this case we have that by definition, the density is given by:
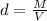
Where:
M: It is the mass of the diamond
V: It is the volume of the diamond
According to the data of the statement we have:
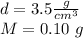
So the volume is:
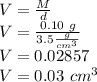
Thus, the volume of the diamond is approximately
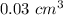
Answer: