Answer: pH of the solution is 3.3
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
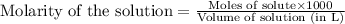 .....(1)
.....(1)
Molarity of HCl solution = 0.0098 M
Volume of solution = 50.0 mL
Putting values in equation 1, we get:
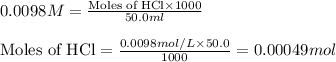
As 1 mole of HCl is giving 1 mole of

0.00049 mole of HCl is giving =
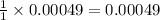 mole of
mole of

pH =
![-log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/nz2oboitfhoilskw38lcd88vzcnql6i9lz.png)
pH =
![-log[0.00049]](https://img.qammunity.org/2021/formulas/chemistry/college/dbffroq92eoym7z0gy8ynfhhuc6p87zt1d.png)
pH=3.3
Thus the pH od the solution is 3.3