Answer:
The total work produced during this process is 24.43 KJ.
Step-by-step explanation:
Here we have from steam tables at 90 °C
P₁ = 0.701824 bar = 70.1824 Pa
Since the quality is 10 %, this gives the fraction of liquid present in the steam, hence we have the specific volume given by
v₁ = 0.00103594 + 0.1 × (2.35915 - 0.00103594) = 0.236847346 m³/kg
When the steam is then heated, we have from the super heated steam tables at T₂ = 250 °C and P₂ = 800 kPa
v₂ =0.293 m³/kg
Therefore the work done is given by
Area under the PV curve, which is
Average pressure × Change in volume
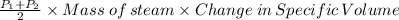
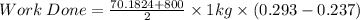
= 24.43 KJ.