Answer: The balanced chemical equation is written below.
Step-by-step explanation:
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
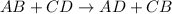
The chemical equation for the reaction of hydrochloric acid and potassium sulfide follows:
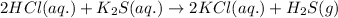
By Stoichiometry of the reaction:
2 moles of hydrochloric acid reacts with 1 mole of potassium sulfide to produce 2 moles of potassium chloride and 1 mole of hydrogen sulfide gas
Hence, the balanced chemical equation is written above.