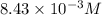Answer: The solubility of calcium sulfate is
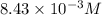
Step-by-step explanation:
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio.
The chemical equation for the ionization of calcium sulfate follows:
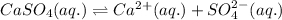
s s
The expression of
 for above equation follows:
for above equation follows:
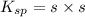
We are given:
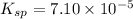
Putting values in above expression, we get:
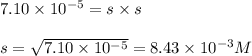
Hence, the solubility of calcium sulfate is