3.88 atm is the pressure in atm, given that there are 1.45 moles of gas in the sample.
Step-by-step explanation:
Given that:
Volume of the gas sample V = 8.77 litres
temperature of the gas sample T = 20 degrees OR 293.15 K
number of moles of the gas = 1.45
R (Gas constant) = 0.08021 L.atm/mole K
Pressure of the gas = ?
The equation for ideal gas will be used here to calculate the pressure of the gas:
PV = nRT
the equation is rewritten as:
P=
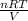
P =
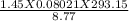
P = 3.88 atm
1.45 moles of the gas at given conditions would have pressure of 3.88 atm.