Answer:
198.8K
Step-by-step explanation:
Given parameters:
Constant volume = constant mole = 50mL
T₁ = 27°C, which gives 27 + 273 = 300K
P₁ = 80kPa
P₂ = 53kPa
T₂ =?
Solution:
Devolving the combined gas law and assuming constant volume, we can solve this problem. The expression obtained will give us a relationship between pressure and temperature.
At constant volume, the pressure of a given mass of gas varies directly with the absolute temperature;
mathematically;
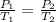
input the parameters and solve for T₂;
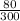 =
=
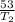
T₂ = 198.8K