Answer: The partial pressure of nitrogen gas in the balloon is 391 mmHg
Step-by-step explanation:
Dalton's law of partial pressure states that the total pressure of the system is equal to the sum of partial pressure of each component present in it.
To calculate the partial pressure of nitrogen gas, we use the law given by Dalton, which is:
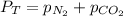
We are given:
Total pressure of the lab,
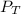 = 770 mmHg
= 770 mmHg
Vapor pressure of carbon dioxide,
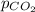 = 379 mmHg
= 379 mmHg
Putting values in above equation, we get:
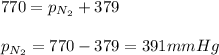
Hence, the partial pressure of nitrogen gas in the balloon is 391 mmHg