Answer: Boyle's law states that gas volume is inversely proportional to pressure.
Step-by-step explanation:
Boyle's law is one of the law used to determine the Ideal Gas equation.
This law states that pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles
Mathematically,
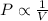 (at constant temperature and number of moles)
(at constant temperature and number of moles)
The above expression can also be written as:
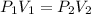
where,
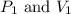 are initial pressure and volume of the gas
are initial pressure and volume of the gas
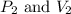 are final pressure and volume of the gas
are final pressure and volume of the gas
Hence, Boyle's law states that gas volume is inversely proportional to pressure.