Answer:
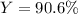
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction:
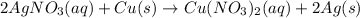
If 4.57 grams of silver nitrate are used in copper excess, the theoretical yield of copper (II) nitrate turns out:

In such a way, the percent yield results:
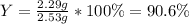
Best regards.