1.35 litres is the volume will the sample have if the pressure changes to 1.50 atm while the temperature is increased to 322 K.
Step-by-step explanation:
Data given:
initial volume in the sample pf gas V1 = 1.42 L
initial temperature of the sample of gas T1 = 293 K
Initial pressure of the gas P1 = 1.30 atm
final pressure of the sample of gas P2= 1.50 atm
final temperature of the sample of gas T2 = 322 K
Final volume V2 = ?
following formula is used:
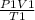 =
=
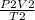
V2 =
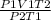
putting the values in the equation:
V2 =
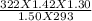
= 1.35 Litre is the volume
When pressure and temperature of the gas is changed the volume becomes 1.35 litres.