Answer:
a) Balanced chemical equation:
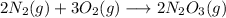
b) Theoretical yield:
c) % yield:
Step-by-step explanation:
The complete question is:
In a particular reaction 6.80g of dinitrogen trioxide gas (N₂0₃) was actually produced by reacting 8.75g of oxygen gas (O₂) with excess nitrogen gas (N₂)
a) Write a balanced chemical equation for the reaction. Be sure to include physical states in the equation.
b) Calculate the theoretical yield (in grams) of dinitrogen trioxide: Use dimensional analysis
c) Calculate the % yield of the product
Solution
a) Write a balanced chemical equation for the reaction. Be sure to include physical states in the equation.
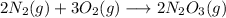
Check the balance:
Atom Left-handside Right-hand side
N 2×2=4 2×2=4
O 3×2=6 2×3=6
- Mole ratio: it is the ratio of the coefficients of the balanced equation
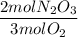
b) Calculate the theoretical yield (in grams) of dinitrogen trioxide: Use dimensional analysis
1. Convert 8.75 g of O₂(g) to number of moles
- number of moles = mass in grams / molar mass
- molar mass of O₂ = 15.999g/mol
- number of moles = 8.75g / 15.999 g/mol = 0.5469 mol O₂
2. Use dimensional analysis to calculate the maximum number of moles of N₂O₃(g) that can be produced
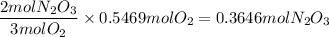
3. Convert to mass in grams
- mass = number of moles × molar mass
- molar mass of N₂O3 = 76.01g/mol
- mass = 0.3646mol × 76.01g/mol = 27.7g N₂O3
c) Calculate the % yield of the product
Formula:
- %yield = (actual yield/theoretical yield)×100
Substitute and compute:
- % yield = (6.80g/27.7g)×100 = 24.5%