Answer:
Step-by-step explanation:
A sound knowledge of specific heat capacity of the metals is required in this case.
The specific heat capacity of a metal is the quantity of heat required to the raise the temperature of a unit mass of it by 1°C.
It is related to quantity of heat using the expression below;
H = m c Δt
where m is the mass
c is the specific heat capacity
Δt is the temperature change
let us make the specific the subject of the expression;
c =
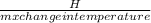
we can see that there is an inverse relationship between specific heat and temperature change.
The specific heat capacity of a body is an intensive property that is unique to the metal.
The higher the specific heat capacity, the lower the amount of temperature change in it.
Let us find the specific heat capacity of the given metals;
Aluminium 0.897J/gK
Iron 0.412J/gK
Silver 0.24J/gK
After the heat is supplied,
Silver > Iron > Aluminium in terms of temperature change