The density of the cube is 0.11 g/cm³
Step-by-step explanation:
Given:
Side of a cube, a = 6 cm
Mass of the cube, m = 24g
Density, d = ?
Volume of the cube = (a)³
V = (6)³
V = 216 cm³
We know,
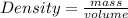
On substituting the value we get:
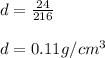
Therefore, the density of the cube is 0.11 g/cm³