9.266 gm/ml is the density of the substance. 9.266 is the specific gravity of the substance.
Step-by-step explanation:
Data given:
mass of the substance = 41.7 grams
initial volume of water = 52.3 ml
volume after adding the substance = 56.8 ml
volume of the metal = 56.8 - 52.3 = 4.5 ml
density =?
specific gravity =?
density is calculated by using the following formula:
density =

=
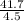
density = 9.266 gm/ml
The ratio of density of the substance with the reference substance density is called specific gravity. In this case the reference substance is water which has density of 1 gm/ml at 4 degrees.
so specific gravity =
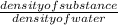
putting values in above equation:
specific gravity =
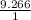
= 9.266 (no units as both denominator and numerator has same units and got cancelled)