The composition of the mixture on a mass basis are as follows:
- He = 131.70 g
- N2 = 1140.41 g
- Ar = 1054.68 g
number of moles = mass of substnce / molar mass
molar mass and molecular weight has equal numerical values therefore
Number of moles = mass of substnce / molecular weight
mass of substance = number of moles * molecular weight
the moles are expressed in percentage hence the total moles should be 100
check: 32.9 + 40.7 + 26.4 = 100
He
mass of substance = 32.9 * 4.003 = 131.6987 g
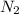
mass of substance = 40.7 * 28.02 = 1140.414 g
Ar
mass of substance = 26.4 * 39.95 = 1054.68 g