Answer:
Kp is 3.03 x 10⁻³ to two significant digits
Step-by-step explanation:
Kp is the ratio of partial pressure of gaseous produce raised to power of their stiochiometric to the partial pressure of gaseous reactant raised to power of their stiochiometric.
For a reaction: aA + bB ==> cC + dD
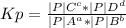
Relating the expression with the given expression and data
A = HCl 76.9 atm with a = 4
B = O₂ 66.3 atm with b = 1
C = Cl₂ 40.7 atm with c = 2
D = H₂O 65.1 atm with d = 2
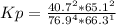
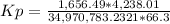
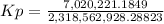
Kp = 0.0030278
Kp = 3.0278 x 10⁻³
Kp is 3.03 x 10⁻³ to two significant digits