Answer:
The
 of acetic acid is 4.7.
of acetic acid is 4.7.
The
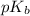 of methylamine is 3.4.
of methylamine is 3.4.
Step-by-step explanation:
1) The dissociation constant of acetic acid =
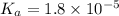
The
 is negative logarithm of dissociation constant.
is negative logarithm of dissociation constant.
![pK_a=-\log[K_a]](https://img.qammunity.org/2021/formulas/chemistry/college/ir8v80n39inyylpe73u5rdi9un2quhm56e.png)
![pK_a=-\log[1.8* 10^(-5)]=4.7](https://img.qammunity.org/2021/formulas/chemistry/high-school/54bob0gvd4mkjtfvxd0caqacfv6b82t0ai.png)
The
 of acetic acid is 4.7.
of acetic acid is 4.7.
2) The dissociation constant of methylamine =
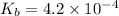
The
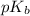 is negative logarithm of dissociation constant.
is negative logarithm of dissociation constant.
![pK_b=-\log[K_b]](https://img.qammunity.org/2021/formulas/chemistry/high-school/e4vngzb96o5c05r299goeai7otaif82kbp.png)
![pK_b=-\log[4.2* 10^(-4)]=3.4](https://img.qammunity.org/2021/formulas/chemistry/high-school/xm65qm19h22yyjw15zvv0rj46w92ludwk6.png)
The
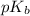 of methylamine is 3.4.
of methylamine is 3.4.