Answer:
The rate of dissolving is equal to the rate of crystallization.
Step-by-step explanation:
In order for a reaction to be at equilibrium, the rate of formation of products of that reaction must be equal to the rate of formation of reactants.
In other words, the rate of forward reaction must be equal to the rate of reverse reaction.
Hence, for the reaction involving the crystallization of solid
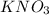 from aqueous
from aqueous
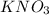 , the rate of dissolution of the solid must be equal to the rate of crystallization.
, the rate of dissolution of the solid must be equal to the rate of crystallization.