Answer:
pH = 5.01
Step-by-step explanation:
The reaction between the sodium propanoate and the HCl added is the following:
CH₃CH₂COO⁻ + H₃O⁺ ⇄ CH₃CH₂COOH + H₂O
initial 0.110M 0.061moles 0.253M
The number of moles of the acid propanoic (a) and sodium propanoate (b) is:
![\eta_(a) = [a]*Va = 0.110 M * 1.41 L = 0.155 moles \thinspace acid](https://img.qammunity.org/2021/formulas/chemistry/high-school/6rmm37v9xuv3wawss75frkqfjepoj1ix7r.png)
![\eta_(b) = [b]*Vb = 0.253 M * 1.41 L = 0.357 moles\thinspace propanoate](https://img.qammunity.org/2021/formulas/chemistry/high-school/r9q7rnaznvstsp6uqqqhv58q92uunnfr4n.png)
After the adding of HCl, the number of moles of acid propanoic and propanoate is:
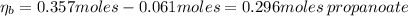
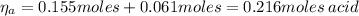
Hence, the pH of the solution after the addition of HCl is:
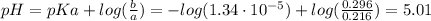
Therefore, the pH of the solution is 5.01.
I hope it helps you!