Answer:
The amount of energy added to rise the temperature Q = 17413.76 KJ
Step-by-step explanation:
Mass of water = 52 kg
Initial temperature
 = 68 °F = 20° c
= 68 °F = 20° c
Final temperature
 = 212 °F = 100° c
= 212 °F = 100° c
Specific heat of water
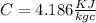
Now heat transfer Q = m × C × (
 -
-
 )
)
⇒ Q = 52 × 4.186 × ( 100 - 20 )
⇒ Q = 17413.76 KJ
This is the amount of energy added to rise the temperature.