Answer:
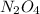 : 0.5 atm and
: 0.5 atm and
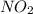 : 1 atm
: 1 atm
Step-by-step explanation:
In order to calculate the partial pressure for each gas , all you need to do is multiply the mole fraction of each gas with the total pressure.
It is given that
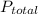 = 1.5 atm
= 1.5 atm
The mole fraction of each gas can be calculated in the following way:
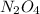 = moles of
= moles of
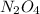 ÷ total moles present =
÷ total moles present =
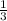
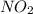 = moles of
= moles of
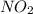 ÷ total moles present =
÷ total moles present =
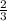
Partial pressure of
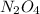 : 1.5 ×
: 1.5 ×
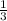 = 0.5 atm
= 0.5 atm
Partial pressure of
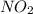 : 1.5 ×
: 1.5 ×
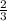 = 1 atm
= 1 atm
Hope that answers the question, have a great day!