Answer:
6.07 mol
Step-by-step explanation:
We know that 1 mole of every substance contains a number of molecules equal to Avogadro Number, which is:
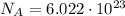
In this problem, we have
 moles of dinitrogen pentoxide, and these n moles contain a number of molecules equal to
moles of dinitrogen pentoxide, and these n moles contain a number of molecules equal to
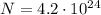
This means that we can find the number of molecules in this substance by using the following rule of three:
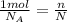
where n is the number of moles that we want to find. Solving for n, we find:
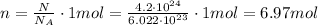
So, the answer is 6.97 moles.