Answer:
3 X ( g ) ⇄ Y ( g ) + Z ( s )
According to le chatelier principle increases in reactant concentration will shift equilibrium towrads right direction.
![([Y])/([X]^(3) )](https://img.qammunity.org/2021/formulas/chemistry/college/k15a0mpyi6tnmsofraa3qyo1rkzgod1idv.png) is constant with change in initial concentration of X.
is constant with change in initial concentration of X.
Step-by-step explanation:
3 X ( g ) ⇄ Y ( g ) + Z ( s )
Equilibrium constant for the reaction is as follows
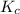 =
=
![([Y])/([X]^(3) )](https://img.qammunity.org/2021/formulas/chemistry/college/k15a0mpyi6tnmsofraa3qyo1rkzgod1idv.png)
At equilibrium there is no change in the ratio
![([Y])/([X]^(3) )](https://img.qammunity.org/2021/formulas/chemistry/college/k15a0mpyi6tnmsofraa3qyo1rkzgod1idv.png) of concentration of products to reactant if initial concentration change.
of concentration of products to reactant if initial concentration change.
Therefore
![([Y])/([X]^(3) )](https://img.qammunity.org/2021/formulas/chemistry/college/k15a0mpyi6tnmsofraa3qyo1rkzgod1idv.png) is constant with change in initial concentration of X.
is constant with change in initial concentration of X.