Answer:
18.0 Ampere is the size of electric current that must flow.
Step-by-step explanation:
Moles of electron , n = 550 mmol = 0.550 mol
1 mmol = 0.001 mol
Number of electrons = N
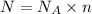
Charge on N electrons : Q
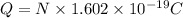
Duration of time charge allowed to pass = T = 49.0 min = 49.0 × 60 seconds
1 min = 60 seconds
Size of current : I
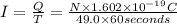
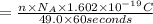
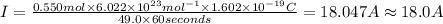
18.0 Ampere is the size of electric current that must flow.