Answer:
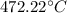
Explanation:
According to the Ideal Gas Law for an isobaric process (at a constant pressure):
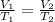 (1)
(1)
Where:
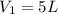 is the initial volume of the sample
is the initial volume of the sample
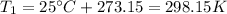 is the initial temperature of the sample in Kelvin
is the initial temperature of the sample in Kelvin
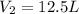 is the final volume of the sample
is the final volume of the sample
 is the final temperature of the sample
is the final temperature of the sample
So, we have to find
 from (1):
from (1):
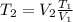 (2)
(2)
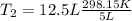 (3)
(3)
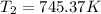 (4)
(4)
Transforming this result to Celsius:
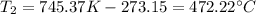 This is the final temperature in Celsius.
This is the final temperature in Celsius.